Free for all! The conditional listing of China’s first Covid-19 vaccine injected confidence into the global victory over the epidemic.
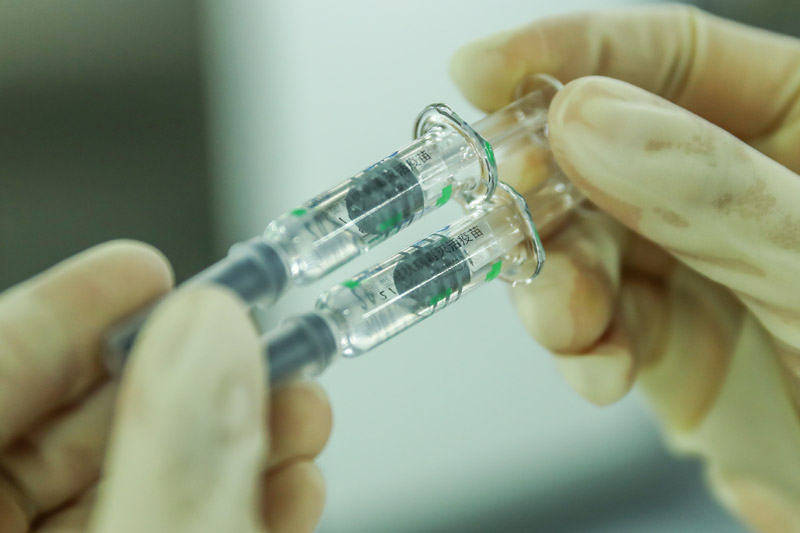
On December 25th, the staff inspected the product packaging quality in the Covid-19 inactivated vaccine sub-packaging workshop of China Institute of Biological Products of Sinopharm Group.
China has taken a crucial step in fighting the COVID-19 epidemic! The joint prevention and control mechanism of the State Council was released on December 31st. The Covid-19 inactivated vaccine of Sinopharm Group China Bio has been approved for conditional marketing in National Medical Products Administration, and its protection effect meets the requirements of relevant standards of the World Health Organization and National Medical Products Administration, and will be provided free of charge for the whole people in the future.
The listing of Covid-19 vaccine in China injected confidence into the global victory over the epidemic and provided strong support for the vaccine to become a global public product.
Xinhua News Agency reporter Zhang Yuwei photo
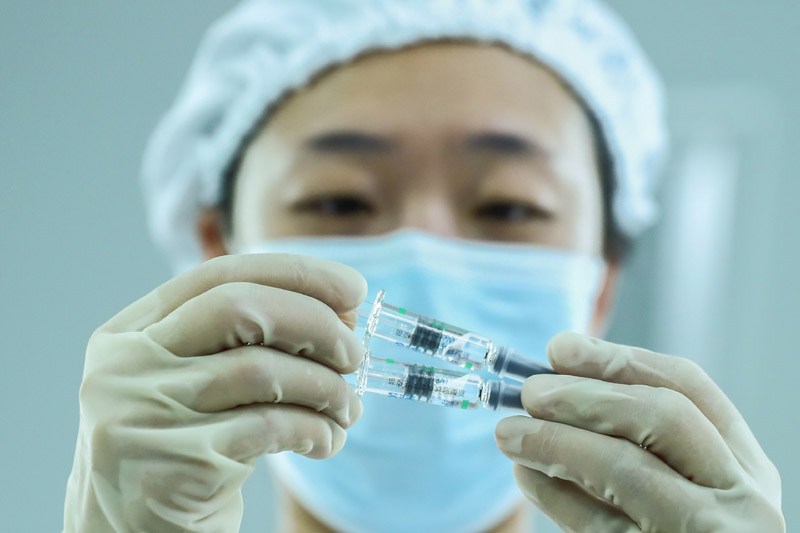
On December 25th, the staff inspected the product packaging quality in the Covid-19 inactivated vaccine sub-packaging workshop of China Institute of Biological Products of Sinopharm Group.
China has taken a crucial step in fighting the COVID-19 epidemic! The joint prevention and control mechanism of the State Council was released on December 31st. The Covid-19 inactivated vaccine of Sinopharm Group China Bio has been approved for conditional marketing in National Medical Products Administration, and its protection effect meets the requirements of relevant standards of the World Health Organization and National Medical Products Administration, and will be provided free of charge for the whole people in the future.
The listing of Covid-19 vaccine in China injected confidence into the global victory over the epidemic and provided strong support for the vaccine to become a global public product.
Xinhua News Agency reporter Zhang Yuwei photo

On December 25th, the staff worked in the Covid-19 Inactivated Vaccine Sub-packaging Workshop of China Institute of Biological Products of Sinopharm Group.
China has taken a crucial step in fighting the COVID-19 epidemic! The joint prevention and control mechanism of the State Council was released on December 31st. The Covid-19 inactivated vaccine of Sinopharm Group China Bio has been approved for conditional marketing in National Medical Products Administration, and its protection effect meets the requirements of relevant standards of the World Health Organization and National Medical Products Administration, and will be provided free of charge for the whole people in the future.
The listing of Covid-19 vaccine in China injected confidence into the global victory over the epidemic and provided strong support for the vaccine to become a global public product.
Xinhua News Agency reporter Zhang Yuwei photo

On December 25th, I photographed the products being packaged in the Covid-19 Inactivated Vaccine Sub-packaging Workshop of China Institute of Biological Products of Sinopharm Group.
China has taken a crucial step in fighting the COVID-19 epidemic! The joint prevention and control mechanism of the State Council was released on December 31st. The Covid-19 inactivated vaccine of Sinopharm Group China Bio has been approved for conditional marketing in National Medical Products Administration, and its protection effect meets the requirements of relevant standards of the World Health Organization and National Medical Products Administration, and will be provided free of charge for the whole people in the future.
The listing of Covid-19 vaccine in China injected confidence into the global victory over the epidemic and provided strong support for the vaccine to become a global public product.
Xinhua News Agency reporter Zhang Yuwei photo

On December 25th, the staff worked in the Covid-19 Inactivated Vaccine Sub-packaging Workshop of China Institute of Biological Products of Sinopharm Group.
China has taken a crucial step in fighting the COVID-19 epidemic! The joint prevention and control mechanism of the State Council was released on December 31st. The Covid-19 inactivated vaccine of Sinopharm Group China Bio has been approved for conditional marketing in National Medical Products Administration, and its protection effect meets the requirements of relevant standards of the World Health Organization and National Medical Products Administration, and will be provided free of charge for the whole people in the future.
The listing of Covid-19 vaccine in China injected confidence into the global victory over the epidemic and provided strong support for the vaccine to become a global public product.
Xinhua News Agency reporter Zhang Yuwei photo

On December 25th, the staff checked the information before shipment from the vaccine cold storage of China Biological Beijing Institute of Biological Products of Sinopharm Group.
China has taken a crucial step in fighting the COVID-19 epidemic! The joint prevention and control mechanism of the State Council was released on December 31st. The Covid-19 inactivated vaccine of Sinopharm Group China Bio has been approved for conditional marketing in National Medical Products Administration, and its protection effect meets the requirements of relevant standards of the World Health Organization and National Medical Products Administration, and will be provided free of charge for the whole people in the future.
The listing of Covid-19 vaccine in China injected confidence into the global victory over the epidemic and provided strong support for the vaccine to become a global public product.
Xinhua News Agency reporter Zhang Yuwei photo

On December 25th, the staff opened the delivery port from the vaccine cold storage of China Institute of Biological Products of Sinopharm Group.
China has taken a crucial step in fighting the COVID-19 epidemic! The joint prevention and control mechanism of the State Council was released on December 31st. The Covid-19 inactivated vaccine of Sinopharm Group China Bio has been approved for conditional marketing in National Medical Products Administration, and its protection effect meets the requirements of relevant standards of the World Health Organization and National Medical Products Administration, and will be provided free of charge for the whole people in the future.
The listing of Covid-19 vaccine in China injected confidence into the global victory over the epidemic and provided strong support for the vaccine to become a global public product.
Xinhua News Agency reporter Zhang Yuwei photo

On December 25th, a cold-chain truck loaded with inactivated Covid-19 vaccine departed from China Institute of Biological Products of Sinopharm Group.
China has taken a crucial step in fighting the COVID-19 epidemic! The joint prevention and control mechanism of the State Council was released on December 31st. The Covid-19 inactivated vaccine of Sinopharm Group China Bio has been approved for conditional marketing in National Medical Products Administration, and its protection effect meets the requirements of relevant standards of the World Health Organization and National Medical Products Administration, and will be provided free of charge for the whole people in the future.
The listing of Covid-19 vaccine in China injected confidence into the global victory over the epidemic and provided strong support for the vaccine to become a global public product.
Xinhua News Agency reporter Zhang Yuwei photo
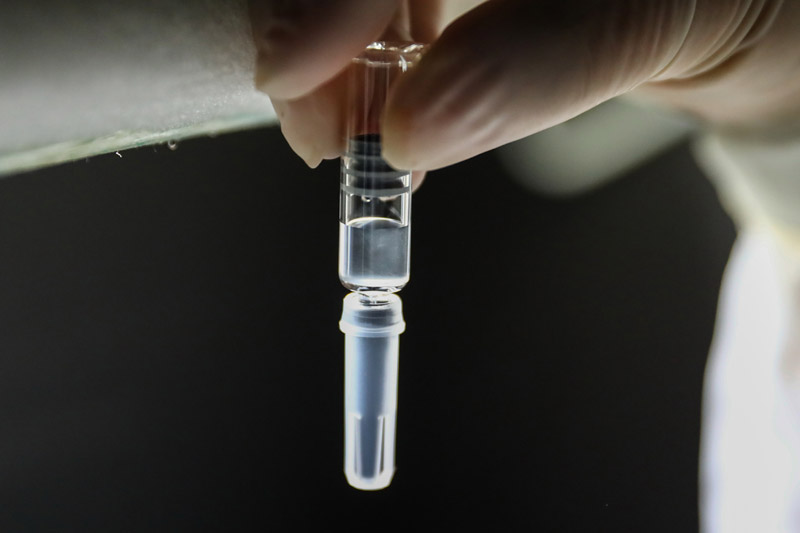
On December 25th, the staff conducted an artificial light inspection in the sub-packaging workshop of Covid-19 inactivated vaccine in China Institute of Biological Products of Sinopharm Group.
China has taken a crucial step in fighting the COVID-19 epidemic! The joint prevention and control mechanism of the State Council was released on December 31st. The Covid-19 inactivated vaccine of Sinopharm Group China Bio has been approved for conditional marketing in National Medical Products Administration, and its protection effect meets the requirements of relevant standards of the World Health Organization and National Medical Products Administration, and will be provided free of charge for the whole people in the future.
The listing of Covid-19 vaccine in China injected confidence into the global victory over the epidemic and provided strong support for the vaccine to become a global public product.
Xinhua News Agency reporter Zhang Yuwei photo
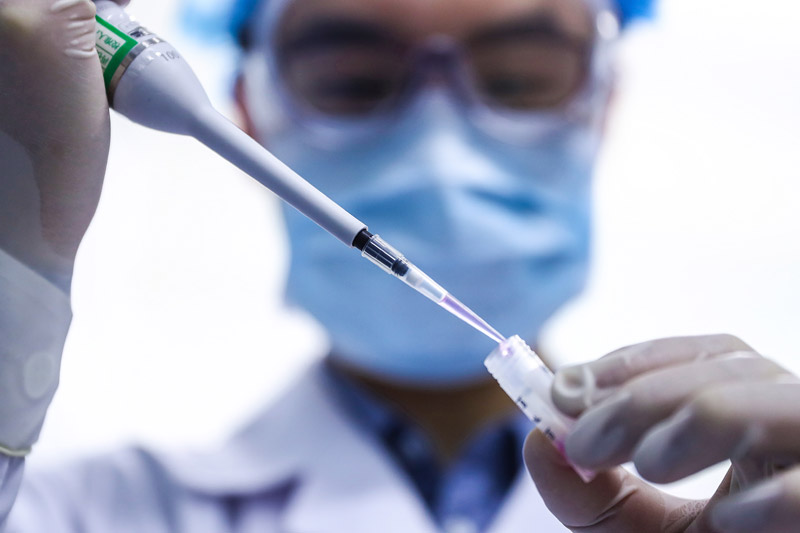
On April 11th, the staff tested the samples of novel coronavirus inactivated vaccine in the quality inspection department of China Bio-COVID-19 Vaccine Production Base of Sinopharm Group.
China has taken a crucial step in fighting the COVID-19 epidemic! The joint prevention and control mechanism of the State Council was released on December 31st. The Covid-19 inactivated vaccine of Sinopharm Group China Bio has been approved for conditional marketing in National Medical Products Administration, and its protection effect meets the requirements of relevant standards of the World Health Organization and National Medical Products Administration, and will be provided free of charge for the whole people in the future.
The listing of Covid-19 vaccine in China injected confidence into the global victory over the epidemic and provided strong support for the vaccine to become a global public product.
Xinhua News Agency reporter Zhang Yuwei photo

On April 10th, the staff cleaned the equipment in the inactivated vaccine production workshop in novel coronavirus, which has not been put into production in China Bio-COVID-19 Vaccine Production Base of Sinopharm Group.
China has taken a crucial step in fighting the COVID-19 epidemic! The joint prevention and control mechanism of the State Council was released on December 31st. The Covid-19 inactivated vaccine of Sinopharm Group China Bio has been approved for conditional marketing in National Medical Products Administration, and its protection effect meets the requirements of relevant standards of the World Health Organization and National Medical Products Administration, and will be provided free of charge for the whole people in the future.
The listing of Covid-19 vaccine in China injected confidence into the global victory over the epidemic and provided strong support for the vaccine to become a global public product.
Xinhua News Agency reporter Zhang Yuwei photo

On April 10th, the staff took a group photo outside the delivery window of novel coronavirus inactivated vaccine production workshop in China Bio-COVID-19 vaccine production base of Sinopharm Group.
China has taken a crucial step in fighting the COVID-19 epidemic! The joint prevention and control mechanism of the State Council was released on December 31st. The Covid-19 inactivated vaccine of Sinopharm Group China Bio has been approved for conditional marketing in National Medical Products Administration, and its protection effect meets the requirements of relevant standards of the World Health Organization and National Medical Products Administration, and will be provided free of charge for the whole people in the future.
The listing of Covid-19 vaccine in China injected confidence into the global victory over the epidemic and provided strong support for the vaccine to become a global public product.
Xinhua News Agency reporter Zhang Yuwei photo
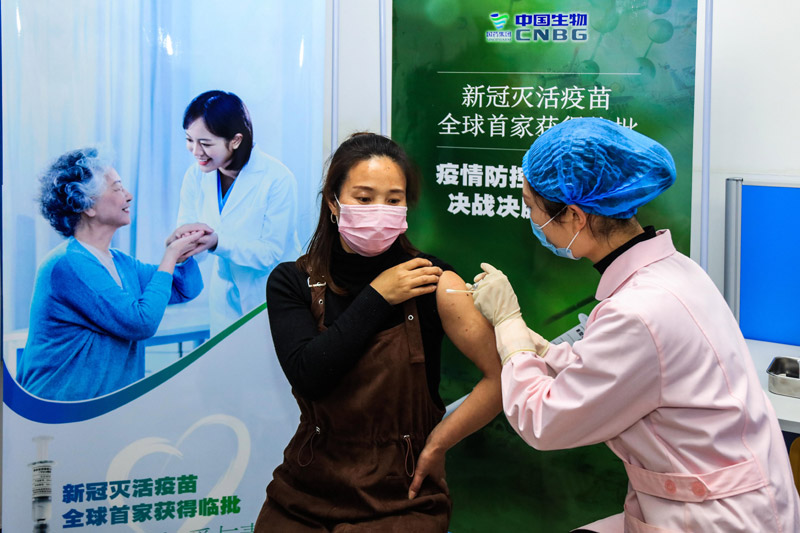
On April 12th, the first volunteer was inoculated with novel coronavirus inactivated vaccine developed by China Institute of Biological Products of Sinopharm Group in Wuzhi County Center for Disease Control and Prevention, Jiaozuo City, Henan Province.
China has taken a crucial step in fighting the COVID-19 epidemic! The joint prevention and control mechanism of the State Council was released on December 31st. The Covid-19 inactivated vaccine of Sinopharm Group China Bio has been approved for conditional marketing in National Medical Products Administration, and its protection effect meets the requirements of relevant standards of the World Health Organization and National Medical Products Administration, and will be provided free of charge for the whole people in the future.
The listing of Covid-19 vaccine in China injected confidence into the global victory over the epidemic and provided strong support for the vaccine to become a global public product.
Xinhua news agency

On June 23rd, the launching ceremony of the International Clinical Phase III of COVID-19 Inactivated Vaccine developed by Sinopharm Group China Biotech was held in the United Arab Emirates.
China has taken a crucial step in fighting the COVID-19 epidemic! The joint prevention and control mechanism of the State Council was released on December 31st. The Covid-19 inactivated vaccine of Sinopharm Group China Bio has been approved for conditional marketing in National Medical Products Administration, and its protection effect meets the requirements of relevant standards of the World Health Organization and National Medical Products Administration, and will be provided free of charge for the whole people in the future.
The listing of Covid-19 vaccine in China injected confidence into the global victory over the epidemic and provided strong support for the vaccine to become a global public product.
Xinhua news agency

On April 11th, the staff tested the samples of novel coronavirus inactivated vaccine in the quality inspection department of China Bio-COVID-19 Vaccine Production Base of Sinopharm Group.
China has taken a crucial step in fighting the COVID-19 epidemic! The joint prevention and control mechanism of the State Council was released on December 31st. The Covid-19 inactivated vaccine of Sinopharm Group China Bio has been approved for conditional marketing in National Medical Products Administration, and its protection effect meets the requirements of relevant standards of the World Health Organization and National Medical Products Administration, and will be provided free of charge for the whole people in the future.
The listing of Covid-19 vaccine in China injected confidence into the global victory over the epidemic and provided strong support for the vaccine to become a global public product.
Xinhua News Agency reporter Zhang Yuwei photo